Surface Segregation Process and Its Influence on High-Temperature Corrosion of Iron-Based Alloys
To protect iron against corrosion, admixtures of other elements are used, but too many admixtures in alloys can lead to many processes unfavourable for the material, e.g. deterioration of mechanical properties.
To limit the amount of additional elements synthesis and processing of the alloy can be designed so that there are many protective dopant atoms on the surface, while iron atoms remain in the volume. This research topic is being intensively developed in our institute by dr Magdalena Sobota, dr Karolina Idczak, dr. eng. Robert Konieczny, and dr. hab. Rafał Idczak, as evidenced by a recent publication in the journal Materials.
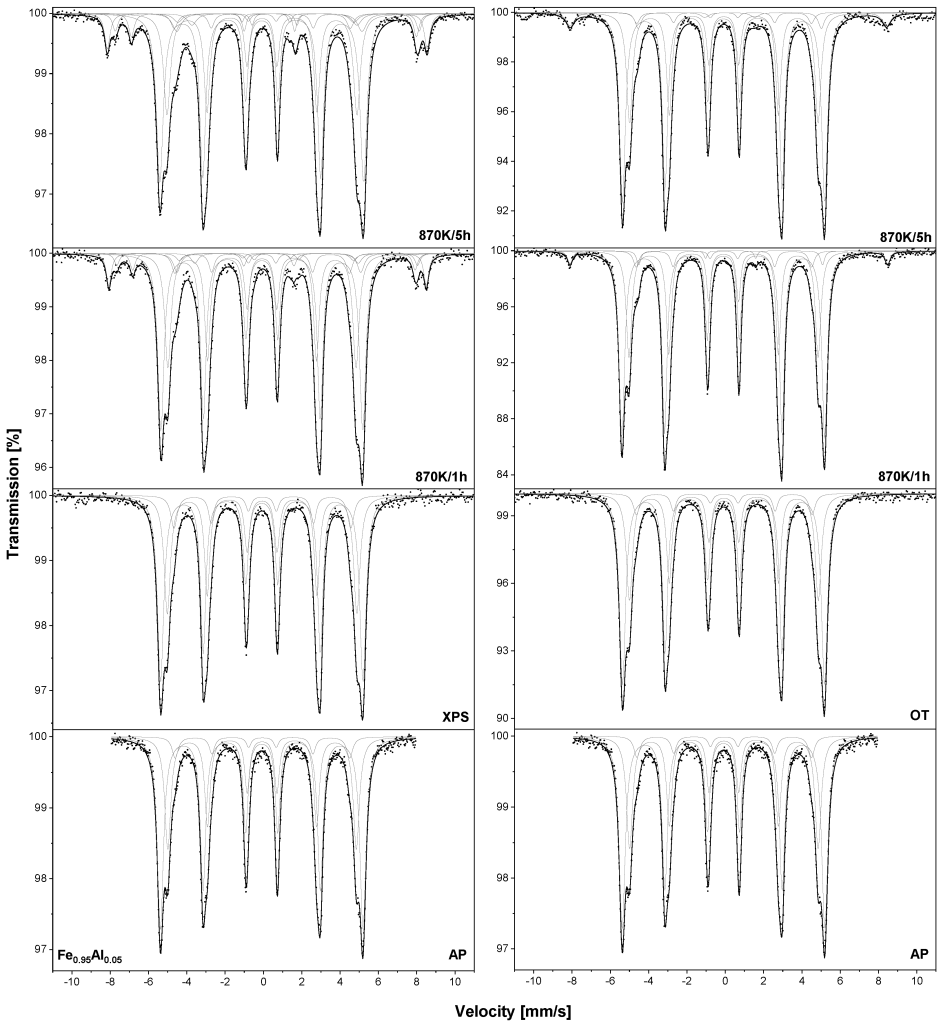
The work demonstrates how different admixtures protect iron against oxidation at high temperatures (Mossbauer spectroscopy performed for samples before and after heat treatment was particularly helpful here) and how those admixtures concentrate on the surface of a sample (here, XPS measurements played a key role). Feel free to read!
The illustration from the publication in question presents TMS spectra at room temperature measured for Fe0,95Al0,05 samples — those annealed at a temperature of 1273 K/15 min in UHV marked as –XPS samples and those annealed in vacuum at a temperature of 933 K/2 h marked as –OT. The samples were oxidized in air at 870 .
